Bio Science
- Home / Bio Science
- MatTek Human 3D Tissue (인공피부)

MatTek’s 3D tissue models of the human airways are advancing respiratory research worldwide. EpiAlveolar was engineered to provide human-relevant results in an easy-to-use in vitro system.
제품 상세정보
EpiAlveolar is a 3D co-culture model of the air-blood barrier. Produced from primary human alveolar epithelial cells, pulmonary endothelial cells and fibroblasts, EpiAlveolar is a in vitro model system developed to mimic human-relevant biological responses. Ready-to-Use EpiAlveolar kits are delivered to our customers in a standard 12-well transwell format and include 24 tissue inserts, maintenance medium, and protocols. EpiAlveolar is used in:


|
A part of the funding for the development of EpiAlveolar was provided |
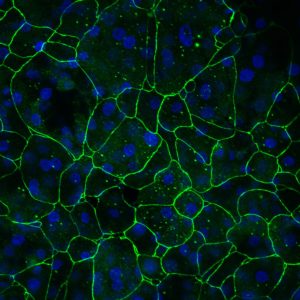
Cross-sectional confocal image. Epithelial cell marker cytokeratin 19 (green), fibroblast marker Vimentin (red) and nuclei marker DAPI (blue)

Kit: A standard EpiAlveolar kit (ALV-100-FT-PE12) consists of 24 tissues. (Tissue “kits” contain tissues, 250 mL of culture medium, and plasticware; contact MatTek for specific kit contents). ALV-100-FT-MAC-PE12, containing THP-1 macrophages is also available.
Formats: 12mm Transwell inserts – tissue culture substrate is chemically modified with a pore size of 0.4 μm
Culture: Air-liquid interface
Histology: 1-2 cell layers – highly squamous morphology and tissue structure.
Lot numbers: Tissue lots produced each week are assigned a specific lot number. A letter of the alphabet is appended to the end of the lot number to differentiate between individual kits within a given lot of tissues. All tissue kits within a lot are identical in regards to cells, medium, handling, culture conditions, etc.
Shipment: At 4°C on medium-supplemented, agarose gels
Shipment Day: ALV-100-FT-PE12 – Every Monday. ALV-100-FT-MAC-PE12 – Every Tuesday.
Delivery: ALV-100-FT-PE12 – Tuesday morning via FedEx priority service (US). Outside US: Tuesday-Wednesday depending on location. ALV-100-FT-MAC-PE12 – Wednesday morning via FedEx priority service (US). Outside US: Wednesday-Thursday depending on location.
Shelf Life: Including time in transit, tissues may be stored at 4°C for up to 3 days prior to use. However, extended storage periods are not recommended. In addition, the best reproducibility will be obtained if tissues are used consistently on the same day.
Length of Experiments: Tissue cultures can be continued for TWO (2) WEEKS or more with good retention of normal morphology. Tissues must be fed every other day with 5.0 ml of maintenance medium (ALV-100-MM). Cell culture inserts are placed into 6-well hanging culture plates provided (HNG-TOP-6-22).
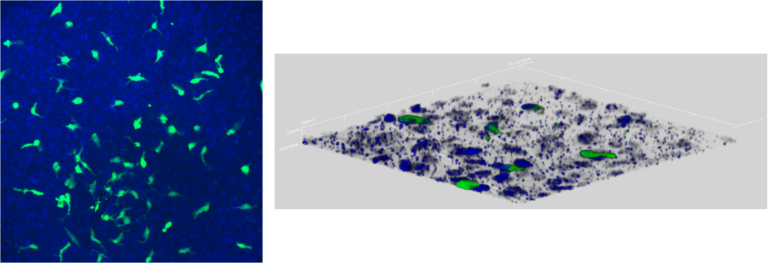
Type: Normal human alveolar epithelial cells (NHAE); Normal human pulmonary fibroblasts (NHPF), Normal human pulmonary endothelial cells (NHPE) and THP-1 macrophages (ALV-100-FT-MAC-PE12).
Genetic make-up: Multiple donors
Derived from: Healthy, non-smoker
Screened for: HIV, Hepatitis-B, Hepatitis-C, mycoplasma
Base medium: Proprietary
Growth factors/hormones: Epidermal growth factors and other proprietary factors
Antibiotics: Gentamicin 30 µg/ml
Anti-fungal agent: Amphotericin B 15 ng/ml
pH Indicator: Phenol red
Other additives: Proprietary
Maintenance medium: ALV-100-MM
Visual inspection: All tissues are visually inspected and if physical imperfections are noted, tissues are rejected for shipment
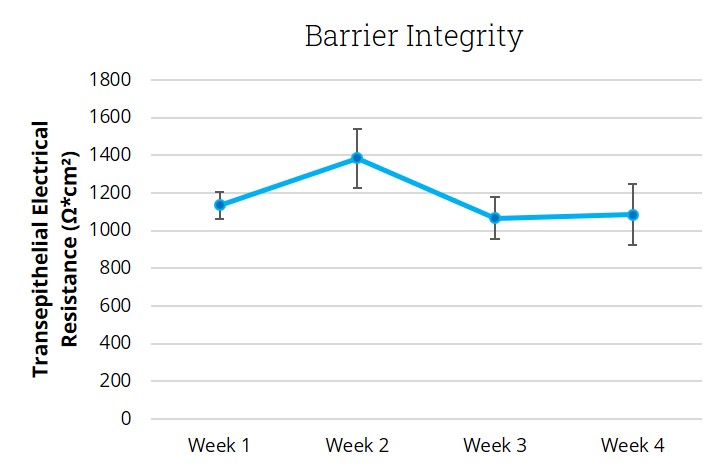
End-use testing: Transepithelial electrical resistance (TEER) of each EpiAlveolar lot is measured using an EVOM2 epithelial Volthommeter and Endohm electrode chamber (World Precision Instruments).
Sterility: All media used throughout the production process is checked for sterility. Maintenance medium is incubated without antibiotics for 1 week and checked for sterility. The agarose gel from the 24-well plate used for shipping is also incubated for 1 week and checked for any sign of contamination
Screening for pathogens: All cells are screened and are negative for HIV, hepatitis B and hepatitis C using PCR. However, no known test method can offer complete assurance that the cells are pathogen-free. Thus, these products and all human-derived products should be handled at BSL-2 levels (biosafety level 2) or higher as recommended in the CDC-NIH manual, “Biosafety in microbiological and biomedical laboratories,” 1998. For further assistance, please contact your site Safety Officer or MatTek technical service
Notification of lot failure: If a tissue lot fails our QC or sterility testing, the customer will be notified and the tissues will be replaced without charge when appropriate. Because our QC and sterility testing is done post-shipment, a notification will be made as soon as possible (Under normal circumstances, TEER failures will be notified by Wednesday 5 p.m.; sterility failures will be notified within 8 days of shipment)

The EpiAlveolar 3D human tissue model is routinely utilized for a range of applications including safety & risk assessment, inflammation & fibrosis, and drug delivery. Simple protocols and the evaluation of early cellular endpoints allow scientists to acquire data in days, not weeks or months.
Use EpiAlveolar to determine relative safety and toxicological potential of inhaled substances.
Use of EpiAlveolar Lung Model to Predict Fibrotic Potential of Multiwalled Carbon Nanotubes
Hana Barosova, Anna G. Maione, Dedy Septiadi, Monita Sharma, Laetitia Haeni, Sandor Balog, Olivia O’Connell, George R. Jackson, David Brown, Amy J. Clippinger, Patrick Hayden, Alke Petri-Fink, Vicki Stone, and Barbara Rothen-Rutishauser. ACS Nano Article ASAP. DOI: 10.1021/acsnano.9b06860
845
Assessment of Alveolar Toxicity and Fibrotic Potential In Vitro using the MatTek EpiAlveolar™ Model
CS Roper, JE Baily, HJ Simpson, and JL Vinall844
844
Organotypic in vitro human airway models can recapitulate aspects of pulmonary fibrosis
Anna G. Maione, George R. Jackson, Olivia O’Connell, Jaclyn Webster, Mitchell Klausner and Patrick J. Hayden758
758
A CO-CULTURE MODEL OF THE AIR-BLOOD (ALVEOLAR) BARRIER RECONSTRUCTED FROM PRIMARY HUMAN CELLS.
Hayden, P., Jackson, G., Mankus, C., Oldach, J., Child, M., Spratt, M. and Ayehunie, S. MatTek Corporation, Ashland, MA 01721 – USA.