Bio Science
- Home / Bio Science
- MatTek Human 3D Tissue (인공피부)

MatTek’s patented EpiDerm system is a leading in vitro testing technology for dermal toxicologists and formulation scientists. With multiple ECVAM validations and OECD accepted test guidelines, EpiDerm is a proven in vitro model system for chemical, pharm
제품 상세정보
MatTek’s patented EpiDerm system is a leading in vitro testing technology for dermal toxicologists and formulation scientists. With multiple ECVAM validations and OECD accepted test guidelines, EpiDerm is a proven in vitro model system for chemical, pharmaceutical and skin care product testing.

Tissue Culture Insert |
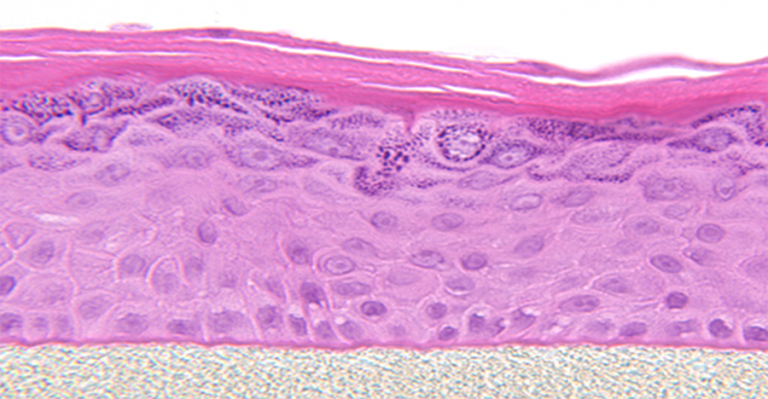
Cultured at the air-liquid interface (ALI), EpiDerm allows for the evaluation of topically applied compounds, chemicals, cosmetic/personal care product ingredients and final formulations. With our straightforward protocols and 20+ years of data, EpiDerm is the gold standard for a broad range of highly predictive in vitro applications. |

Kit: A standard EpiDerm kit (EPI-200) consists of 24 tissues. (Tissue “kits” contain tissues, a small amount of culture medium, and plasticware; contact MatTek for specific kit contents)
Formats: 9mm & 22mm individual inserts and 96-well HTS plates – tissue culture substrate is chemically modifies with a pore size of 0.4 μm
Culture: Air-liquid interface
Histology: 8-12 cell layers plus stratum corneum (basal, spinous, and granular layers)
Lot numbers: Tissue lots produced each week are assigned a specific lot number. A letter of the alphabet is appended to the end of the lot number to differentiate between individual kits within a given lot of tissues. All tissue kits within a lot are identical in regards to cells, medium, handling, culture conditions, etc.
Shipment: At 4°C on medium-supplemented, agarose gels
Shipment day: Every Monday. Shipment on Thursday also possible upon special request
Delivery: Tuesday morning via FedEx priority service (US). Outside US: Tuesday-Thursday depending on location
Shelf life: Including time in transit, tissues may be stored at 4°C for up to 4 days prior to use. However, extended storage periods are not recommended unless necessary. In addition, the best reproducibility will be obtained if tissues are used consistently on the same day, e.g. Tuesday afternoon or following overnight storage at 4°C (Wednesday morning)
Length of experiments: Cultures can be continued for at least 2 weeks with good retention of normal epidermal morphology. Cultures must be fed every other day with 5.0 mL of either New Maintenance Medium (EPI-100-NMM-3) or EPI-100-NMM-113. Cell culture inserts are placed hang top plates (HNG-TOP-12, provided) to allow the use of 5.0 mL.
Type: Normal human epidermal keratinocytes (NHEK)
Genetic make-up: Single donor
Derived from: Neonatal-foreskin tissue (NHEK)
Alternatives: NHEK from adult breast skin
Screened for: HIV, Hepatitis-B, Hepatitis-C, mycoplasma
Base medium: Dulbecco’s Modified Eagle’s Medium (DMEM)
Growth factors/hormones: Epidermal growth factor, insulin, hydrocortisone and other proprietary stimulators of epidermal differentiation
Serum: None
Antibiotics: Gentamicin 5 µg/ml (10% of normal gentamicin level)
Anti-fungal agent: Amphotericin B 0.25 µg/ml
pH Indicator: Phenol red
Other additives: Lipid precursors used to enhance epidermal barrier formation (proprietary)
Alternatives: Phenol red-free (EPI-200-PRF), antibiotic-free (EPI-200-ABF), anti-fungal-free (EPI-200-AFF), or hydrocortisone-free medium and tissue (EPI-200-HCF) are available. Agents are removed at least 3 days prior to shipment. Please discuss your specific media formulation and tissue needs with MatTek scientific personnel before placing your order.
Assay/Maintenance medium: Three maintenance media are available. EpiDerm maintenance medium (EPI-100-MM) is equivalent to the assay medium (EPI-100-ASY) and is used for short-term toxicological testing (≤ 24 hrs). New Maintenance Medium, EPI-100-NMM, contains Keratinocyte Growth Factor (KGF) and is used for studies up to 3 days. EPI-100-NMM-3 contains higher levels of KGF and is used for longer-term studies (> 3 days). For good retention of epidermal morphology for at least 2 weeks, the use of EPI-100-NMM-3 (5.0 mL changed every other day) is recommended.
Visual inspection: All tissues are visually inspected and if physical imperfections are noted, tissues are rejected for shipment
End-use testing: Tissues are exposed to 1% Triton X-100 for 4, 6, 8 and 12.5 hours. The time of exposure required to reduce the tissue viability (ET-50) using the MTT viability assay is determined (See MatTek EpiDerm MTT ET-50 Protocol) for each lot of tissue. ET-50’s must fall within the range of the 1996 EpiDerm database of 4.77 – 8.72 hours. ET-50’s in customers’ labs may differ slightly from the MatTek results.
Sterility: All media used throughout the production process is checked for sterility. Maintenance medium is incubated with and without antibiotics for 1 week and checked for sterility. The agarose gel from the 24-well plate used for shipping is also incubated for 1 week and checked for any sign of contamination
Screening for pathogens: All cells are screened and are negative for HIV, hepatitis B and hepatitis C using PCR. However, no known test method can offer complete assurance that the cells are pathogen free. Thus, these products and all human derived products should be handled at BSL-2 levels (biosafety level 2) or higher as recommended in the CDC-NIH manual, “Biosafety in microbiological and biomedical laboratories,” 1998. For further assistance, please contact your site Safety Officer or MatTek technical service
Notification of lot failure: If a tissue lot fails our QC or sterility testing, the customer will be notified and the tissues will be replaced without charge when appropriate. Because our QC and sterility testing is done post-shipment, notification will be made as soon as possible (Under normal circumstances, ET-50 failures will be notified by Wednesday 5 p.m.; sterility failures will be notified within 8 days of shipment)

The EpiDerm 3D human tissue model is used across a diverse range of applications including safety and risk assessment, and biological efficacy. With Air-liquid Interface (ALI) culture technology, EpiDerm can be used to evaluate biological responses to topical applications of formulations or ingredients. Simple protocols and the evaluation of early cellular endpoints allow research to acquire data in days, not weeks or months.
Use EpiDerm to determine the skin irritation potential of liquids, solids, gels, lotions, ointments, or creams. Tissue viability results are acquired within 3 days.
Requesting the Skin Irritation Test Protocol
Determine the skin corrosion potential of test materials in a single day.
Requesting the Skin Corrosion Protocol
Measure the electrical impedance of EpiDerm to assess the efficacy of topically applied moisturizers including creams, gels, and lotions. The Skin Hydration protocol allows for data analysis within 24 hours.
Skin Hydration Application Note
Utilize existing transdermal permeation equipment or MatTek’s simple single insert permeation devices to assess API permeation and flux. Determine relative safety with the EpiDerm MTT ET-50 assay.
Requesting the Percutaneous Absorption Protocol
Identify phototoxic effects of topically applied test substances using the EpiDerm Phototoxicity Assay.
Requesting the Phototoxicity Protocol
Identify the effects of systemically applied test substances using the EpiDerm tissue model.
Requesting the Systemic Phototoxicity Protocol
Identify genotoxic effects of topical or systemic test substances using EpiDerm Genotoxicity Assays (pre-validated)
Comet Assay Protocol
Evaluate active compounds for effects on epidermal differentiation using the under-developed EpiDerm tissue model
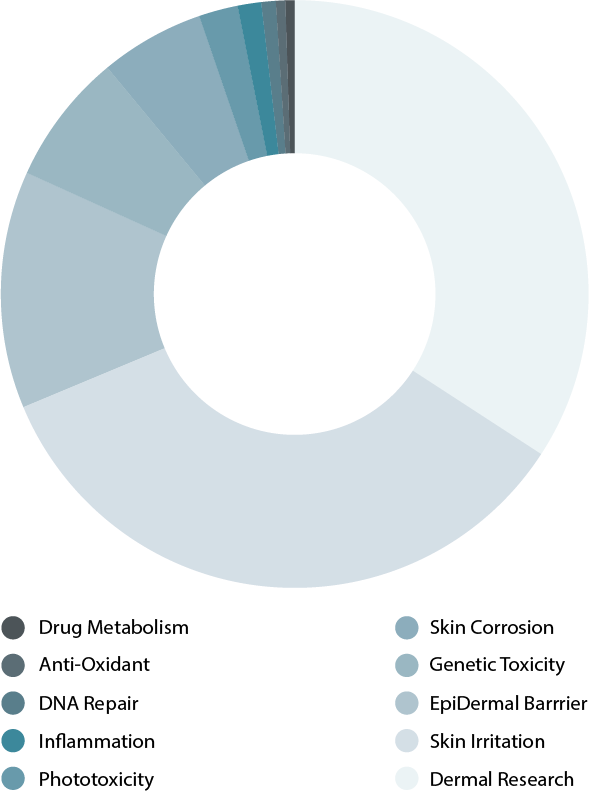
Browse our reference library to see how our EpiDerm tissue has been used in these areas of study.
We have you covered.
With multiple ECVAM validations and OECD accepted test guidelines, EpiDerm is a proven in vitro model system for chemical, pharmaceutical and skin care product testing.