Bio Science
- Home / Bio Science
- MatTek Human 3D Tissue (인공피부)

An in vitro 3D diseased tissue model of Psoriasis to screen therapeutic candidates for safety and efficacy.
제품 상세정보
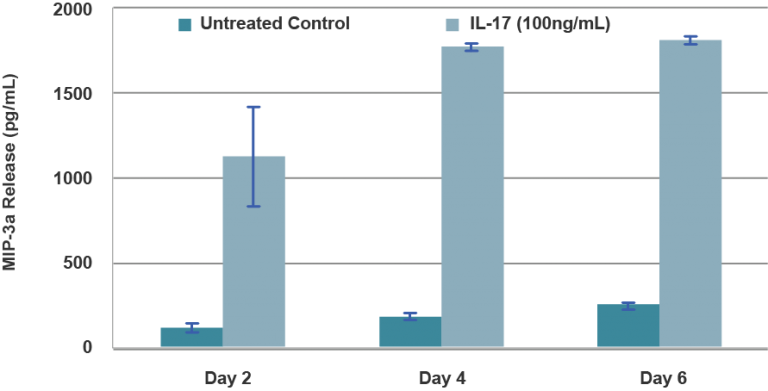
Psoriasis is a chronic inflammatory skin disease characterized by epidermal hyperplasia (Acanthosis) with elongated ridges and abnormal differentiation of epidermal keratinocytes, dermal angiogenesis, abnormal accumulation of polymorphonuclear leukocytes, infiltration of activated T cells and dendritic cells, and increased cytokine levels. MatTek has developed in vitro model of Psoriasis to enable the study of the disease, and to screen therapeutic candidates for safety and efficacy.


Kit: The Psoriasis Tissue Kit (SOR-300-FT) consists of 24 tissues.
Substrate: Millipore Millicell™ Cell culture insert used. Pore size = 0.4 μm, Diameter = 0.8 cm, Surface area = 0.6 cm2.
Culture: At air liquid interface (apical tissue surface exposed to the atmosphere).
Histology: 8-12 cell layers plus stratum corneum (basal, spinous, and granular layers).
Lot Numbers: Tissue lots are produced every other week and are assigned a specific lot number. A letter of the alphabet is appended to the end of the lot number to differentiate between individual kits within a given lot of tissues. All tissue kits within a lot are identical in regards to cells, medium, handling, culture conditions, etc.
Shipment: At 2-8°C on medium-supplemented, agarose gels in 24-well plates.
Shipment day: Every other Monday.
Delivery: Tuesday morning via FedEx priority service (US). Outside US: Tuesday-Thursday depending on location.
Shelf life: Including time in transit, tissues may be stored in their original package at 2-8°C for up to 4 days prior to use. The best reproducibility will be obtained if tissues are used consistently on the same day, e.g. Tuesday afternoon or following overnight storage at 2-8°C (Wednesday morning). Extended storage periods are not recommended.
Length of experiments: Cultures can be continued for up to 2 weeks with good retention of normal epidermal morphology. Cultures must be fed every other day with 5.0 ml of SOR-300-FT-MM medium. Culture stands (MatTek part #: MEL-STND) are required for experiments in excess of 24 hrs.
SOR-300-FT-ABF: Antibiotic-free tissue. For last 3 days of culture, gentamicin is omitted from culture medium. Customer also receives SOR-300-FT-MM-AFAB medium.
SOR-300-FT-AFF: Anti-fungal-free tissue. For last 3 days of culture, Amphotericin is omitted from culture medium. Customer also receives SOR-300-FT-MM-AFAB medium.
SOR-300-FT-AFAB: Antibiotic, anti-fungal-free tissue. For last 3 days of culture, gentamicin and Amphotericin are omitted from culture medium. Customer also receives SOR-300-FT-MM-AFAB medium.
SOR-300-FT-CTR: Normal healthy control tissue for psoriasis experiments
Type: Normal human epidermal keratinocytes (NHEK); psoriatic human dermal fibroblasts (PHDF).
Derived from: Neonatal-foreskin tissue (NHEK); adult psoriatic explants (PHDF)
Screened for: HIV, Hepatitis-B, Hepatitis-C, and mycoplasma.
Base medium: Dulbecco’s Modified Eagle’s Medium (DMEM).
Growth factors/hormones: Epidermal growth factor, insulin, hydrocortisone and other proprietary stimulators of epidermal differentiation.
Serum: None.
Antibiotics: Gentamicin 5 µg/ml (10% of the normal gentamicin level).
Anti-fungal agent: Amphotericin B 0.25 µg/ml.
pH Indicator: Phenol red.
Other additives: Lipid precursors used to enhance epidermal barrier formation (proprietary).
Alternatives: Phenol red-free (SOR-300-FT-PRF), antibiotic-free (SOR-300-FT-ABF), anti-fungal-free (SOR-300-FT-AFF), or hydrocortisone-free medium and tissue (SOR-300-FT-HCF) are available. Agents are removed 3 days prior to shipment.
Assay/Maintenance medium: SOR-300-FT-MM is used for equilibration of tissues following shipment and for maintenance of the SOR-300-FT tissues in culture during experiments.
Visual inspection: All tissues are visually inspected and if physical imperfections are noted, tissues are rejected for shipment.
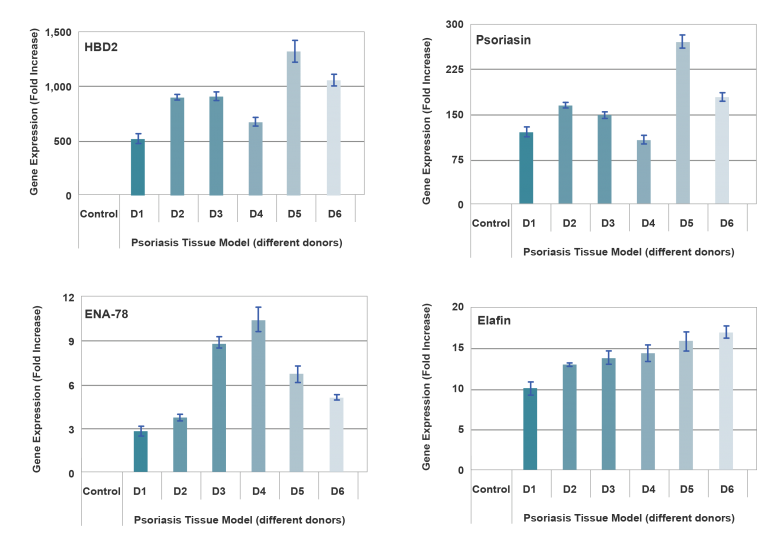
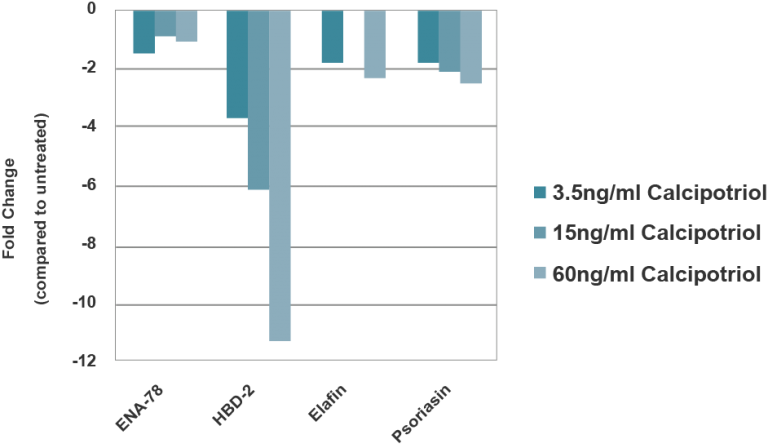
End-use testing: Tissues are exposed to 1% Triton X-100 for 4, 6, 8 and 10 hours. The time of exposure required to reduce the tissue viability (ET-50) using the MTT viability assay is determined (See MatTek Psoriasis Tissue use protocol) for each lot of tissue. ET-50’s generally fall within the range of 6.0-12.0 hours. ET-50’s in customers’ lab may differ slightly from the MatTek results. In addition, qPCR data for psoriasis related genes will be monitored. Increases of >5 fold for psoriasin, human beta-defensin-2 and elafin are expected.
Sterility: All media used throughout the production process is checked for sterility. Maintenance medium is incubated with and without antibiotics for 1 week and checked for sterility. The agarose gel from the 24-well plate used for shipping is also incubated for 1 week and checked for any sign of contamination.
Screening for pathogens: All cells are screened and are negative for HIV, hepatitis B and hepatitis C using PCR. However, no known test method can offer complete assurance that the cells are pathogen free. Thus, these products and all human derived products should be handled at BSL-2 levels (biosafety level 2) or higher as recommended in the CDC-NIH manual, “Biosafety in microbiological and biomedical laboratories,” 1998. For further assistance, please contact your site Safety Officer or MatTek technical service.
Notification of lot failure: If a tissue lot fails our QC or sterility testing, the customer will be notified and the tissues will be replaced without charge when appropriate. Because our QC and sterility testing is done post-shipment, notification will be made as soon as possible (Under normal circumstances, ET-50 failures will be notified by Thursday 5 pm; sterility failures will be notified within 8 days of shipment).

The model provides researchers with a useful, in vitro means to assess the pre-clinical safety and efficacy of lead therapeutic compounds.
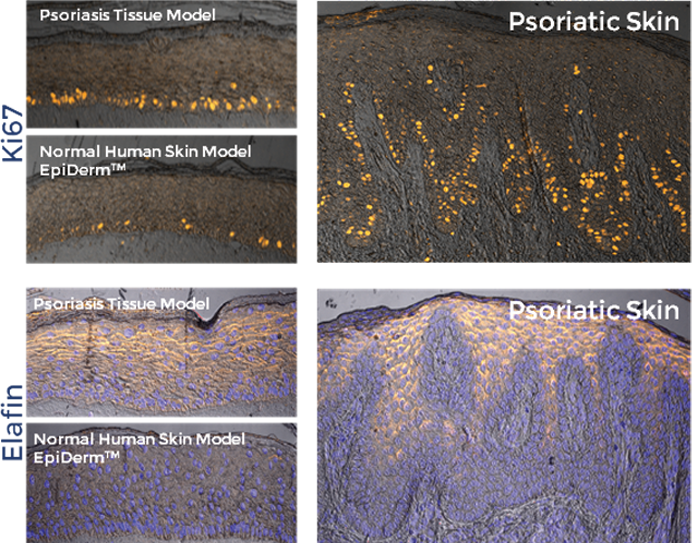
The tissue model closely parallels lesional psoriatic human tissues in terms of morphology and cytokine expression. This model provides researchers with a useful, in vitro means to study psoriasis biology phenomena.
Search our library of technical papers for more information.
Understand the disease, and test new medicines
|
MatTek’s psoriasis model mimics the morphology and progression of psoriasis in vivo, making it a valuable tool for the study of the disease, and evaluation of the efficacy of therapeutic candidates. |
DELPHINIDIN INDUCES EPIDERMAL DIFFERENTIATION AND INHIBITS PROLIFERATION AND INFLAMMATION IN A THREE-DIMENSIONAL RECONSTITUTED SKIN MODEL OF PSORIASIS.
Pal1, H.C., Sharma1, S., Ayehunie2, S. and Afaq1, F. 1Department of Dermatology, University of Alabama at Birmingham, Birmingham, AL, USA 2MatTek Corporation, 200 Homer Avenue, Ashland, MA, USA