Bio Science
- Home / Bio Science
- MatTek Human 3D Tissue (인공피부)
EpiVaginal provides an in vitro research tool for feminine product safety testing, drug development and for the study of HIV-1 and other sexually transmitted diseases. Human-like 3D tissue structure and cellular physiology make it ideal for toxicology, dr
제품 상세정보
EpiVaginal provides an in vitro research tool both for feminine product safety testing and for the study of HIV-1 and other sexually transmitted diseases. Its tissue structure and cellular physiology closely parallel in vivo vaginal epithelial tissue, making it ideal for toxicology and efficacy testing, as well as a tool for biological research.

EpiVaginal tissues are cultured from normal, human-derived vaginal epithelial and dendritic cells. Its highly-differentiated structure parallels in vivo tissue and is ideal for toxicity studies of feminine hygiene, vaginal care, and microbicide products. Four types of EpiVaginal are offered:
1) VEC-100 (Figure 1): An epithelial tissue containing epithelial VEC cells,
2) VLC-100 (Figure 1): An epithelial tissue containing epithelial VEC and immuno-competent dendritic cells,
3) VEC-100-FT (Figure 2): A full-thickness version of VEC-100 which includes VEC epithelial cells and a fibroblast-containing lamina propria, and
4) VLC-100-FT (Figure 2): An immuno-competent version of the VEC-100-FT which includes dendritic cells.
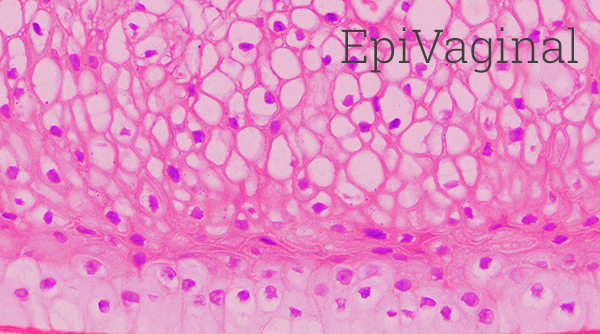
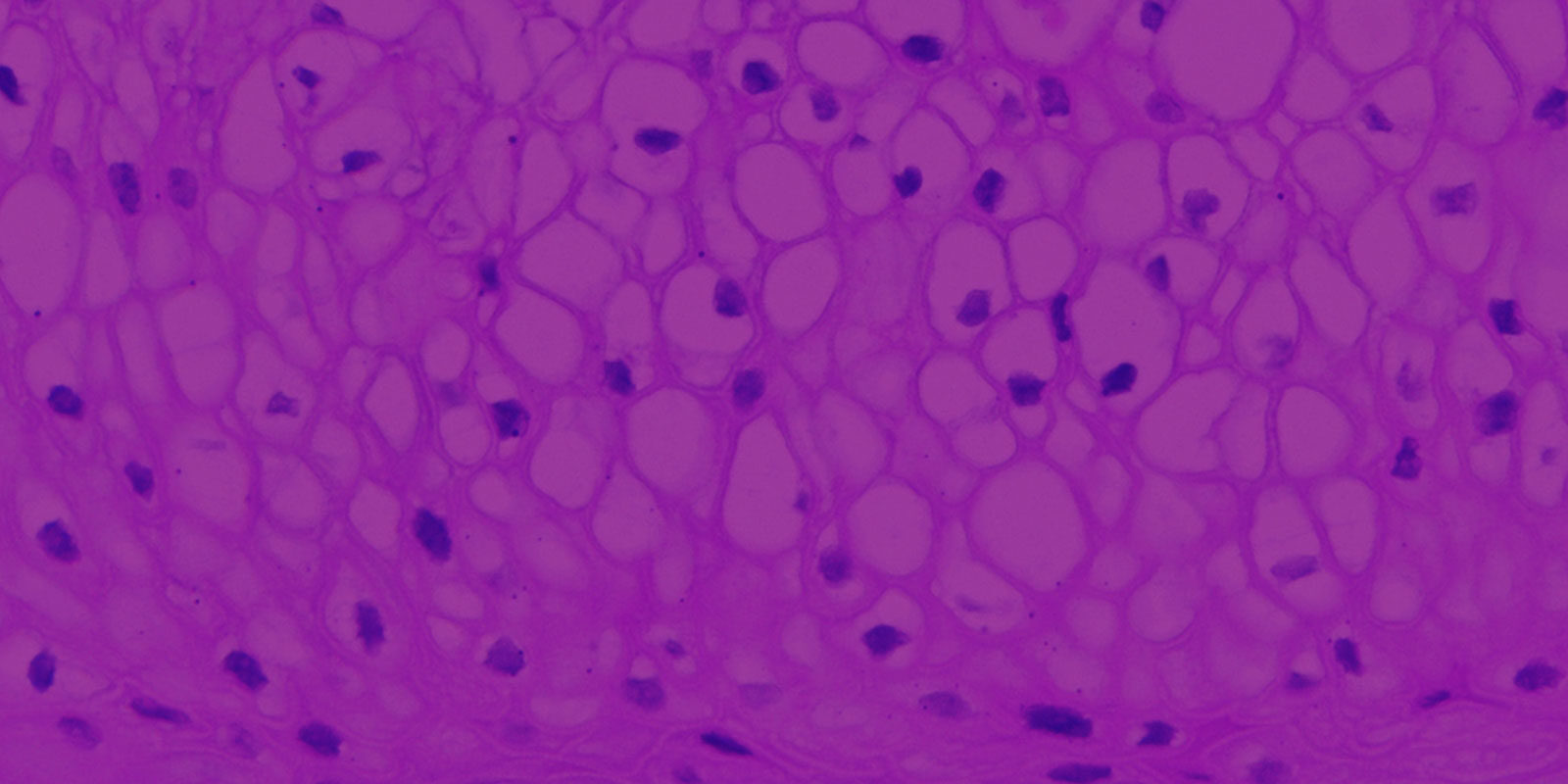
Figure 1: H&E stained histological (formalin-fixed) cross-sections of A) VEC-100 in vitro reconstructed epithelial tissue models containing normal human VEC cells, and B) vaginal explant tissue. Both in vitro and in vivo tissues show nucleated basal and suprabasal cell layers followed by layers in which nuclei are lost and cells are filled with glycogen.
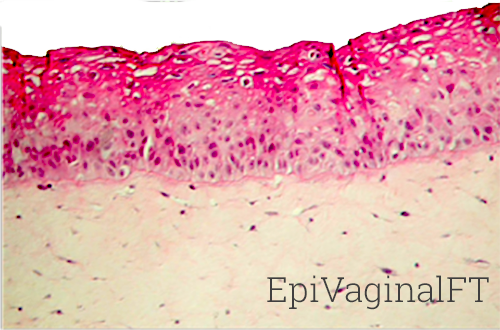
Figure 2: H&E stained histological (formalin-fixed) cross-sections of full-thickness EpiVaginal tissues. The VEC-100-FT tissues consist of VEC epithelial cells cultured atop a lamina propria (LP)-like collagen matrix that contains fibroblasts. The VLC-100-FT consists of VEC epithelial and dendritic cells in the epithelial layers and contains both fibroblasts and dendritic cells in the LP.
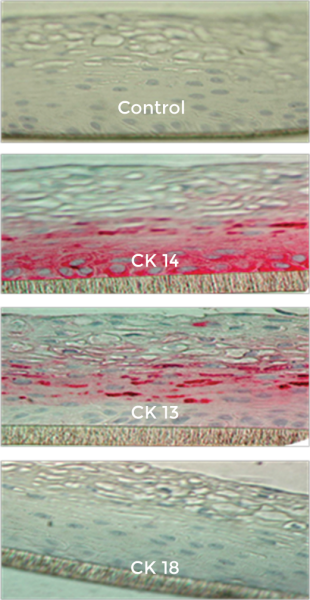
Figure 3: Formalin-fixed cross-sections of the VEC-100 tissue immuno-stained for cytokeratins, CK13, CK14, & CK18. Basal cells are stained by CK14 and supra-basal cells by CK13; no cells are stained by CK18.
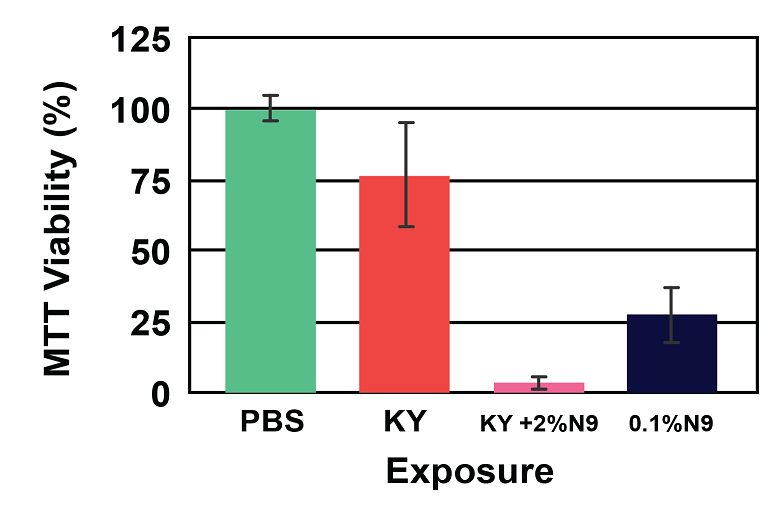
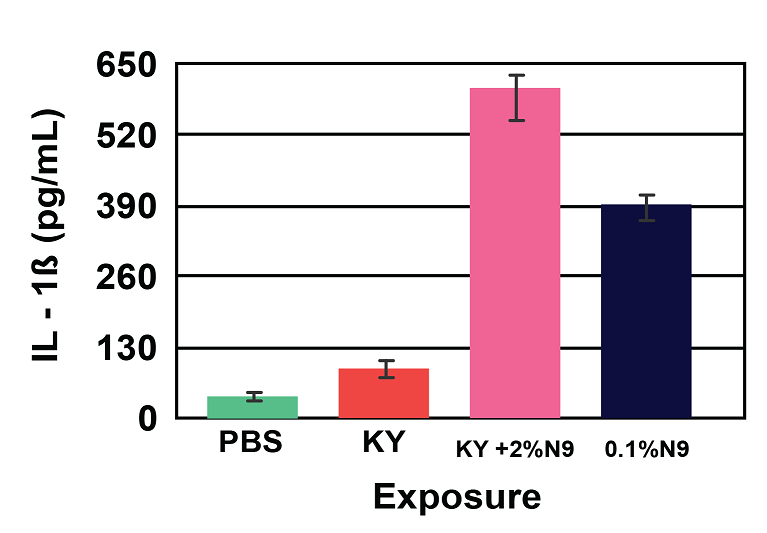
Figure 4: Viability (A) and Cytokine Release (B) of EpiVaginal full-thickness (VEC-100-FT) tissue model following 18 hr exposure to formulations containing the common spermicide, nonoxynol-9 (N9): a) PBS control, b) KY jelly (KY), c) KY with 2% N9 (2% N9), and d) 0.1% N9 in PBS (0.1% N9). Note: As the tissue viability decreases, the IL-1β release increases.
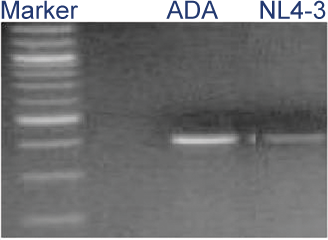
Figure 5: HIV-1 infection of VLC-100 tissue following topical exposure (24hr) to 60,000 CPM of HIV-1 ADA or NL4-3 virus. After exposure, tissues were washed 3X and then cultured for an additional 24 hr. Cellular DNA was then extracted and HIV-1 transcripts were detected using HIV-1 gag specific primer pairs.
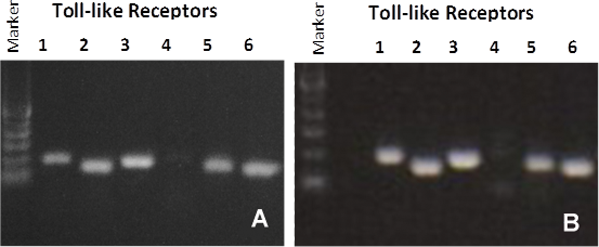
Figure 6: RT PCR showing Toll-like Receptor (TLR) expression for: A) organotypic vaginal-ectocervical (VEC) tissue model and B) Human cervical tissue explant.

Kits: The EpiVaginal kit VEC-100 contains 24 tissues, VEC-112 contains 12 tissues and VEC-606 contains 6 tissues. (Tissue “kits” contain tissues, a small amount of culture medium, and plasticware; contact MatTek for specific kit contents.)
Substrate: Single well tissue culture plate inserts are used.
VEC-100: Nunc™ single well tissue culture plate inserts. Pore Size = 0.4 µm, Inner Diameter = 0.80 cm, Surface Area = 0.5 cm2.
VEC-606: Polycarbonate single well tissue culture plate inserts, Nunc™. Pore Size = 0.4 µm, Inner Diameter = 2.5 cm, Surface Area = 4.2 cm2.
Culture: At air liquid interface.
Histology: VEC-100: 10-16 cell layers of non-cornified tissue.
Lot Numbers: Tissue lots produced by each technician for each week are assigned a specific lot number. Typically, there are multiple lot numbers for any given week’s tissue production. A letter of the alphabet is appended to the end of the lot number to differentiate between individual kits within a given lot of tissues. All tissue kits within a lot are identical with regard to cells, medium, handling, culture conditions, etc.
Shipment: At 4°C on medium-supplemented, agarose gels.
Shipment day: Monday.
Delivery: Tuesday morning via FedEx priority service (US). Outside US: Tuesday-Thursday depending on location.
Shelf life: Including time in transit, tissues may be stored at 4°C for up to 4 days prior to use. However, extended storage periods are not recommended unless absolutely necessary. In addition, the best reproducibility will be obtained if tissues are used consistently on the same day, e.g. Wednesday morning following overnight storage at 4°C.
Length of experiments: Cultures can be continued for at least 1 week with good retention of normal morphology. Cultures must be fed every other day with 5.0 ml of maintenance medium (VEC-100-MM). Cell culture inserts are placed atop culture stands (part number MEL-STND) or washers (part number EPI-WSHR) in 6-well plates to allow the use of 5.0 ml.
Alternative tissues:
VEC-100-FT: Similar to VEC-100 except tissue grown on a collagen/vaginal fibroblast substrate (lamina propria).
VEC-112-FT: Similar to VEC-100-FT except 12 tissues.
VLC-100: Similar to VEC-100 except tissues contain Langerhans cells.
VLC-112: Similar to VLC-100 except 12 tissues.
VLC-100-FT: Similar to VLC-100 except tissue grown on a collagen/vaginal fibroblast substrate (lamina propria).
VLC-112-FT: Similar to VLC-100-FT except 12 tissues.
Type: Normal human ectocervical cells (NHEC) are differentiated into tissues with a non-cornified, vaginal-ectocervical phenotype (part number VEC-100).
Genetic make-up: Single donor.
Derived from: adult human ectocervical tissue.
Screened for: HIV, Hepatitis-B, Hepatitis-C, mycoplasma, bacteria, yeast, fungi.
Base medium: Dulbecco’s Modified Eagle’s Medium (DMEM) and F12 Medium.
Growth factors/hormones: Epidermal growth factor and other proprietary factors.
Serum: None.
Antibiotics: Gentamicin 5 µg/ml (10% of normal gentamicin level).
Anti-fungal agent: Amphotericin B 0.25 µg/ml.
pH Indicator: Phenol red.
Other additives: Proprietary.
Alternatives: Phenol red-free, antibiotic-free, anti-fungal-free medium and tissue are available. Agents are removed 3 days prior to shipment.
Maintenance medium: VEC-100-MM
Visual inspection: All tissues are visually inspected and if physical imperfections are noted, tissues are rejected for shipment.
End-use testing: Tissue viability of each lot is determined by an MTT assay. Tissues are exposed to 1.0% Triton X-100 for 30, 60, and 120 minutes. The time of exposure required to reduce the tissue viability (ET-50) using the MTT assay is determined. The time of exposure needed to reduce the viability to 50% (ET-50) must be 0.92 – 1.67 hours. A summary of the QC testing results will be provided to the customer for reference upon request. (For TEER measurements, MatTek has successfully used the WPI Endohm System.)
Sterility: All media used throughout the production process is checked for sterility. Maintenance medium is incubated with and without antibiotics for 1 week and checked for sterility. The agarose gel from the 24-well plate used for shipping is also incubated for 1 week and checked for any sign of contamination.
Screening for pathogens: All cells are screened and are negative for HIV, hepatitis B and hepatitis C using PCR. However, no known test method can offer complete assurance that the cells are pathogen free. Thus, these products and all human derived products should be handled at BSL-2 levels (biosafety level 2) or higher as recommended in the CDC-NIH manual, “Biosafety in microbiological and biomedical laboratories,” 1998. For further assistance, please contact your site Safety Officer or MatTek technical service.
Notification of lot failure: If a tissue lot fails our QC or sterility testing, the customer will be notified and the tissues will be replaced without charge when appropriate. Because some of our QC and sterility testing is done post-shipment, notification will be made as soon as possible. (Under normal circumstances, ET-50 failures will be notified by Thursday, 5 p.m.; sterility failures will be notified within 8 days of shipment.)

Microbicide Testing, STD Infection Research, Feminine Hygiene, Inflammation, Drug Delivery
Use the EpiVaginal tissue to measure the tissue viability and cytokine release following exposure to feminine hygiene and other products.
EpiVaginal Irritation Protocol
EpiVaginal provides an in vitro research tool both for feminine product safety testing and for the study of HIV-1 and other sexually transmitted diseases. Its tissue structure and cellular physiology closely parallel in vivo vaginal epithelial tissue, making it ideal for toxicology and efficacy testing, as well as a tool for biological research. Browse our reference library to see how researchers have used our EpiVaginal tissue in these areas of study.
In a non-animal system
Avoid the variability and late-stage failures of in vivo testing. Assess the irritancy of surfactant-based vaginal products in the developmental stage, using EpiVaginal and a simple in vitro assay. |
|