Bio Science
- Home / Bio Science
- MatTek Human 3D Tissue (인공피부)
EpiOcular’s exceptional reproducibility provides scientists with quantifiable assessments of irritation. Its correlation to in vivo sensitivity makes it an ideal in vitro alternative to in vivo testing for toxicological evaluation of raw materials and fin
제품 상세정보
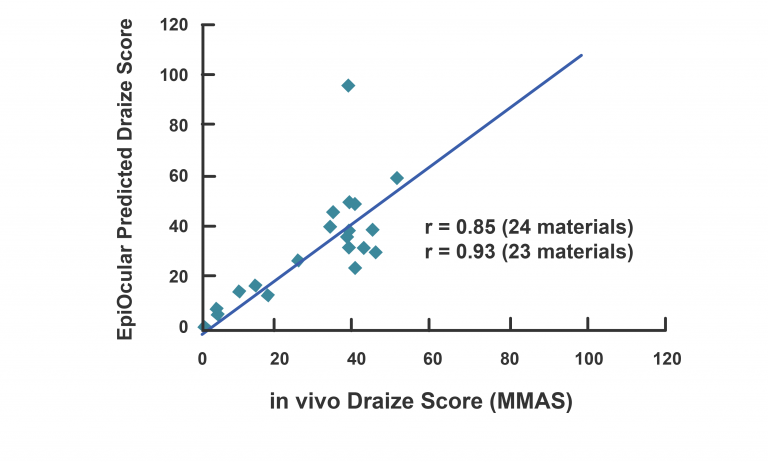
EpiOcular’s strong correlation to historical in vivo data sets and exceptional reproducibility make it an ideal model for the ocular safety testing of raw ingredients and final formulations. The EpiOcular Eye Irritation Test (OECD TG 492) is an in vitro alternative to animal testing and is a validated OECD test guideline (TG 492). EpiOcular has been used for many years by industry as a non-animal, in vitro alternative to determine Draize scores (for water-soluble test articles) and to assess the mildness / ultra-mildness (sub-Draize) of materials contacting the eyes.


Kit: Standard EpiOcular™ kit (OCL-200) consists of 24 tissues, each tissue 9 mm in diameter. (Tissue “kits” contain tissues, a small amount of culture medium, and plasticware; contact MatTek for specific kit contents.)
Substrate: Non-coated, 9 mm ID single well tissue culture plate inserts are used (e.g. Millicell PCF, Nunc polycarbonate single well tissue culture plate inserts).
Culture: At air liquid interface.
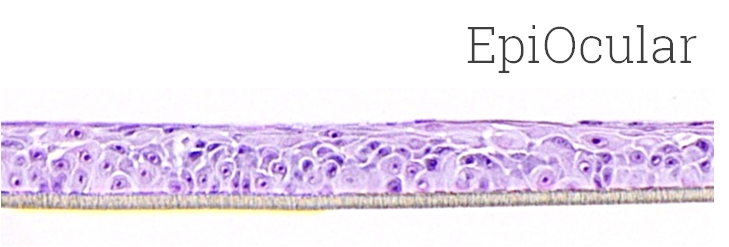
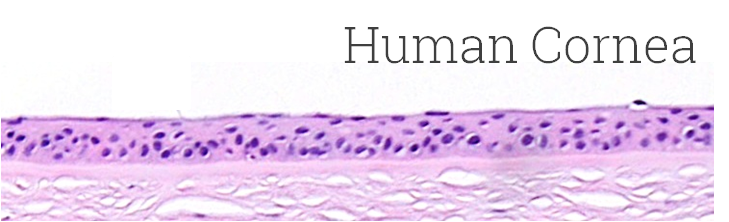
Histology: 5-8 cell layers (basal layer to apical surface).
Cornification: None.
Lot Numbers: Tissue lots produced by each technician for each week are assigned a specific lot number. Typically, there are multiple lot numbers for any given week’s tissue production. A letter of the alphabet is appended to the end of the lot number to differentiate between individual kits within a given lot of tissues. All tissue kits within a lot are identical in regards to cells, medium, handling, culture conditions, etc.
Shipment: At 4°C on medium-supplemented, agarose gels in 24-well plate.
Shipment day: Every Monday. Shipment on Thursday also possible upon special request.
Delivery: Tuesday morning via FedEx priority service (US). Outside US: Tuesday-Thursday depending on location.
Shelf life: Including time in transit, tissues may be stored at 4°C for up to 4 days prior to use. However, extended storage periods are not recommended unless absolutely necessary. In addition, the best reproducibility will be obtained if tissues are used consistently on the same day, e.g. Tuesday afternoon or following overnight storage at 4°C (Wednesday morning).
OCL-200-ABF: Antibiotic-free tissue. For last 3 days of culture, gentamicin is omitted from culture medium. Customer receives 200 ml of OCL-200-ASY-AFAB medium.
OCL-200-AFF: Anti-fungal-free tissue. For last 3 days of culture, Amphotericin is omitted from culture medium. Customer receives 200 ml of OCL-200-ASY-AFAB medium.
OCL-200-AFAB: Antibiotic, anti-fungal-free tissue. For last 3 days of culture, gentamicin and Amphotericin are omitted from culture medium. Customer receives 200 ml of OCL-200-ASY-AFAB medium.
OCL-200-EACH: Single EpiOcular tissue insert.
OCL-200-FRZN-EA: Single OCL-200 tissue that has been subjected to 3 freeze/thaw cycles. Designed to serve as control for MTT auto-reduction test.
OCL-212: Half kit (12 cultures instead of standard 24).
OCL-606: Same as OCL-200 except 6 tissues per kit, each tissue cultured in a larger cell culture insert (22 mm diameter).
Type: Normal human epidermal keratinocytes (NHEK).
Genetic make-up: Single donor.
Derived from: Neonatal-foreskin tissue.
Screened for: HIV, Hepatitis-B, Hepatitis-C, mycoplasma.
Base medium: Dulbecco’s Modified Eagle’s Medium (DMEM).
Growth factors/hormones: Epidermal growth factor, insulin, hydrocortisone and other proprietary stimulators of non-keratinizing epithelial differentiation.
Serum: None.
Antibiotics: Gentamicin 5 µg/ml (10% of normal gentamicin level).
Anti-fungal agent: Amphotericin B 0.25 µg/ml.
pH Indicator: Phenol red.
Alternatives: Phenol red-free, antibiotic-free, anti-fungal-free, or hydrocortisone-free medium and tissue are available. Agents are removed at least 3 days prior to shipment.
Maintenance medium: Most assays with EpiOcular™ are performed within 24 hours. However, for longer experiments, the tissue can be maintained in OCL-200-MM (identical to EpiOcular™ assay medium).
Visual inspection: All tissues are visually inspected and if physical imperfections are noted, tissues are rejected for shipment.
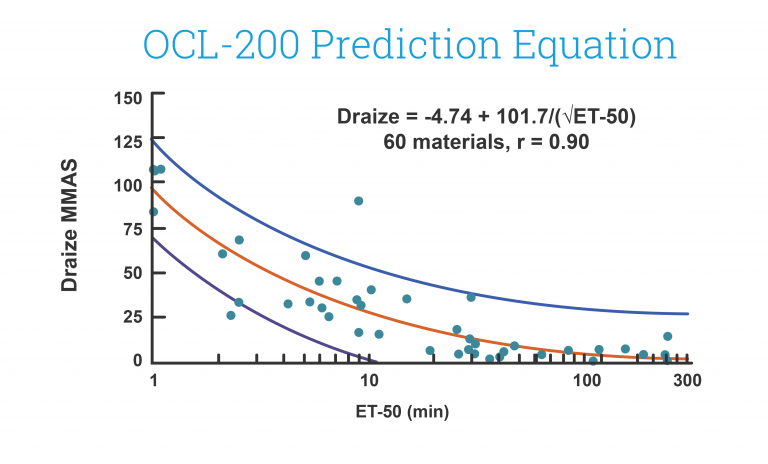
End-use testing: Tissues are exposed to 0.3% Triton X-100 for 5, 20, and 60 minutes. The time of exposure required to reduce the tissue viability (ET-50) using the MTT assay is determined (See MatTek EpiOcularTM ET-50 protocol) for each lot of tissue. ET-50’s must fall within the range of the 1996 EpiOcular™ database: 12.2-37.5 minutes. ET-50’s in customers’ labs are often very similar to MatTek results.
Sterility: All media used throughout the production process is checked for sterility. Maintenance medium is incubated with and without antibiotics for 1 week and checked for sterility. The agarose gel from the 24-well plate used for shipping is also incubated for 1 week and checked for any sign of contamination.
Screening for pathogens: All cells are screened and are negative for HIV, hepatitis B and hepatitis C using PCR. However, no known test method can offer complete assurance that the cells are pathogen free. Thus, these products and all human derived products should be handled at BSL-2 levels (biosafety level 2) or higher as recommended in the CDC-NIH manual, “Biosafety in microbiological and biomedical laboratories,” 1998. For further assistance, please contact your site Safety Officer or MatTek technical service.
Notification of lot failure: If a tissue lot fails our QC or sterility testing, the customer will be notified and the tissues will be replaced without charge. Because our QC and sterility testing is done post-shipment, notification will be made as soon as possible (Under normal circumstances, ET-50 failures will be notified by Wednesday 5pm; sterility failures will be notified within 8 days of shipment).

The EpiOcular EIT is an in vitro hazard assessment assay that has the ability to differentiate materials that are ocular non-irritants from materials that are ocular irritants. The EIT is applicable to a broad range of organic and inorganic chemicals and has regulatory acceptance as OECD TG 492.
EpiOcular Eye Irritation Test Protocol
Watch a video on how to perform the EpiOcular Eye Irritation Test (EIT – OECD TG 492):
The EpiOcular Ultra-mildness Test is an in vitro risk assessment assay routinely utilized for mild materials for which the Draize test is insensitive. This highly reproducible assay allows for quantifiable discrimination among mild, milder, and mildest product formulations.
This in vitro risk assessment assay is based on the effective time at which a material (applied neat) causes a 50% reduction in tissue viability (ET-50). Based on the ET-50, the test article is categorized into one of 4 classifications ranging from non-irritating to severe/extreme which correspond to groupings of Rabbit Draize Eye Scores (MMAS).
EpiOcular MTT ET-50 Neat Protocol
Similar to the MTT ET-50 Neat method, this in vitro risk assessment assay is based on the effective time at which a material (diluted to 20% in water) causes a 50% reduction in tissue viability (ET-50). Based on the ET-50, the test article is categorized into one of 4 classifications ranging from non-irritating to severe/extreme which correspond to groupings of Rabbit Draize Eye Scores (MMAS). This method is recommended for surfactant-based solutions and is applicable to water-soluble materials with a specific gravity of > 0.95.
EpiOcular MTT ET-50 Dilution Protocol
Browse our reference library to see how our EpiOcular tissue has been used in these areas of study.
The most reliable in vitro ocular model on the market
With years of reproducible reliability, and a recent OECD accepted test guideline, EpiOcular is a proven non-animal method for chemical, pharmaceutical and personal care product testing. |
|